Encube Ethicals Job Vacancy For M.Pharm
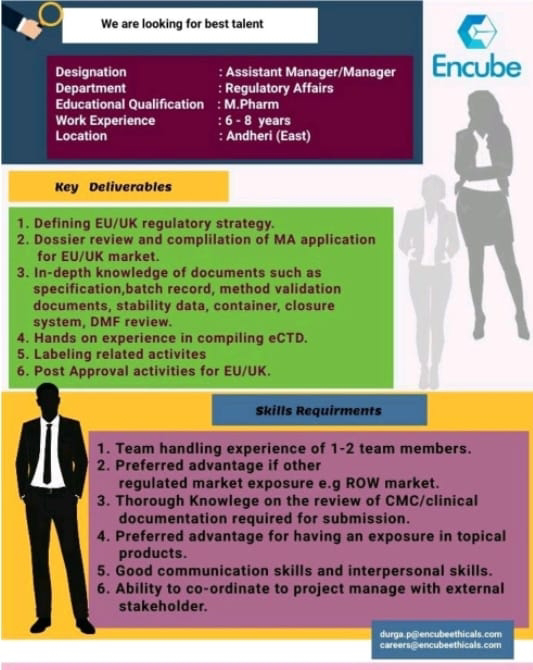
We are looking for best talent
Department: Regulatory Affairs
Designation: Assistant Manager/Manager
Educational Qualification: M.Pharm
Work Experience:6-8 years
Location :Andheri (East)
Key Deliverables
1. Defining EU/UK regulatory strategy.
2. Dossier review and complilation of MA application for EU/UK market.
3. In-depth knowledge of documents such as specification, batch record, method validation documents, stability data, container, closure system, DMF review.
4. Hands on experience in compiling eCTD.
5. Labeling related activites 6. Post Approval activities for EU/UK.
Skills Requirments
1. Team handling experience of 1-2 team members.
2. Preferred advantage if other regulated market exposure e.g ROW market.
3. Thorough Knowlege on the review of CMC/clinical documentation required for submission.
4. Preferred advantage for having an exposure in topical products.
5. Good communication skills and interpersonal skills.
6. Ability to co-ordinate to project manage with external stakeholder.
Post a Comment